- Water is the most common substance on Earth, covering about 71% of the planet’s surface.
- Water is the only substance that exists naturally in all three physical states of matter: solid, liquid, and gas.
- Water is a universal solvent, meaning it can dissolve a variety of substances, including salts, acids, and bases.
- Water has a unique density anomaly, which means it becomes less dense as it freezes causing ice to float on water.
- Water has a high specific heat capacity, meaning it can absorb and store a large amount of heat energy before its temperature increases significantly.
- Water is essential for all known forms of life and plays a vital role in many biological processes, such as hydration, metabolism, and waste removal.
- Water can form hydrogen bonds, which are weak electrostatic attractions between water molecules, giving it unique properties such as surface tension and capillary action.
- Water has a pH of 7, which is considered neutral. Acidic solutions have a lower pH value, and basic solutions have a higher pH value.
- Water can be split into hydrogen and oxygen through a process called electrolysis, making it a potential source of clean energy.
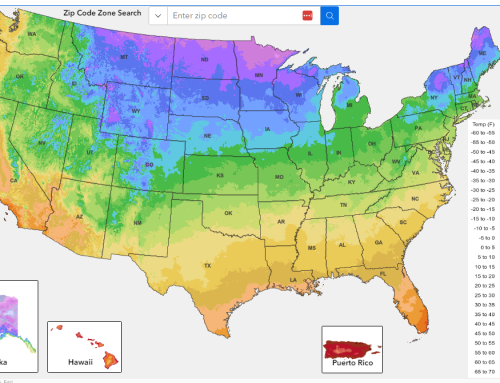
- Water is constantly cycling through the Earth’s atmosphere, oceans, rivers, and lakes, in a process known as the water cycle, which helps to regulate the planet’s temperature and climate.